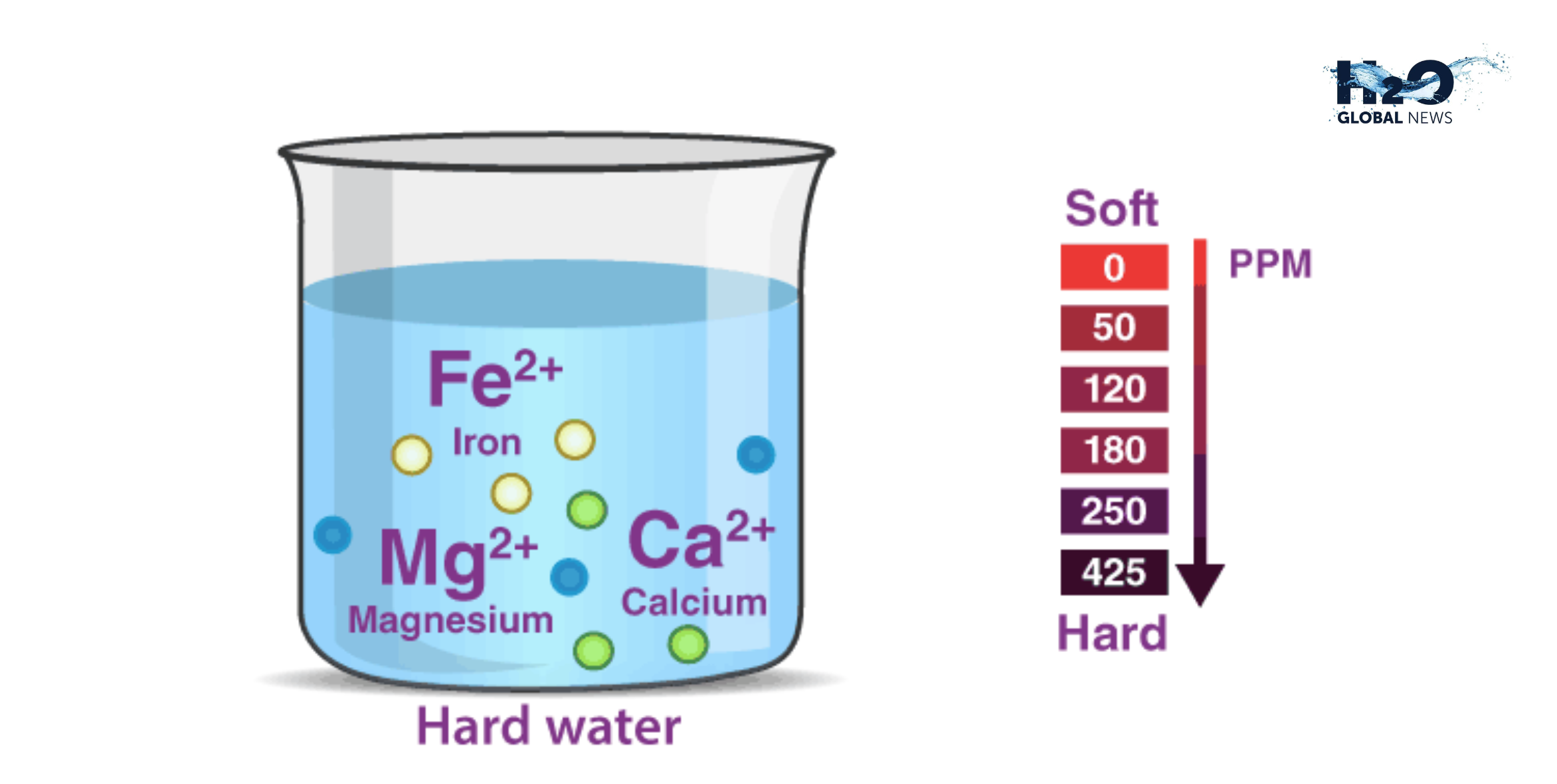
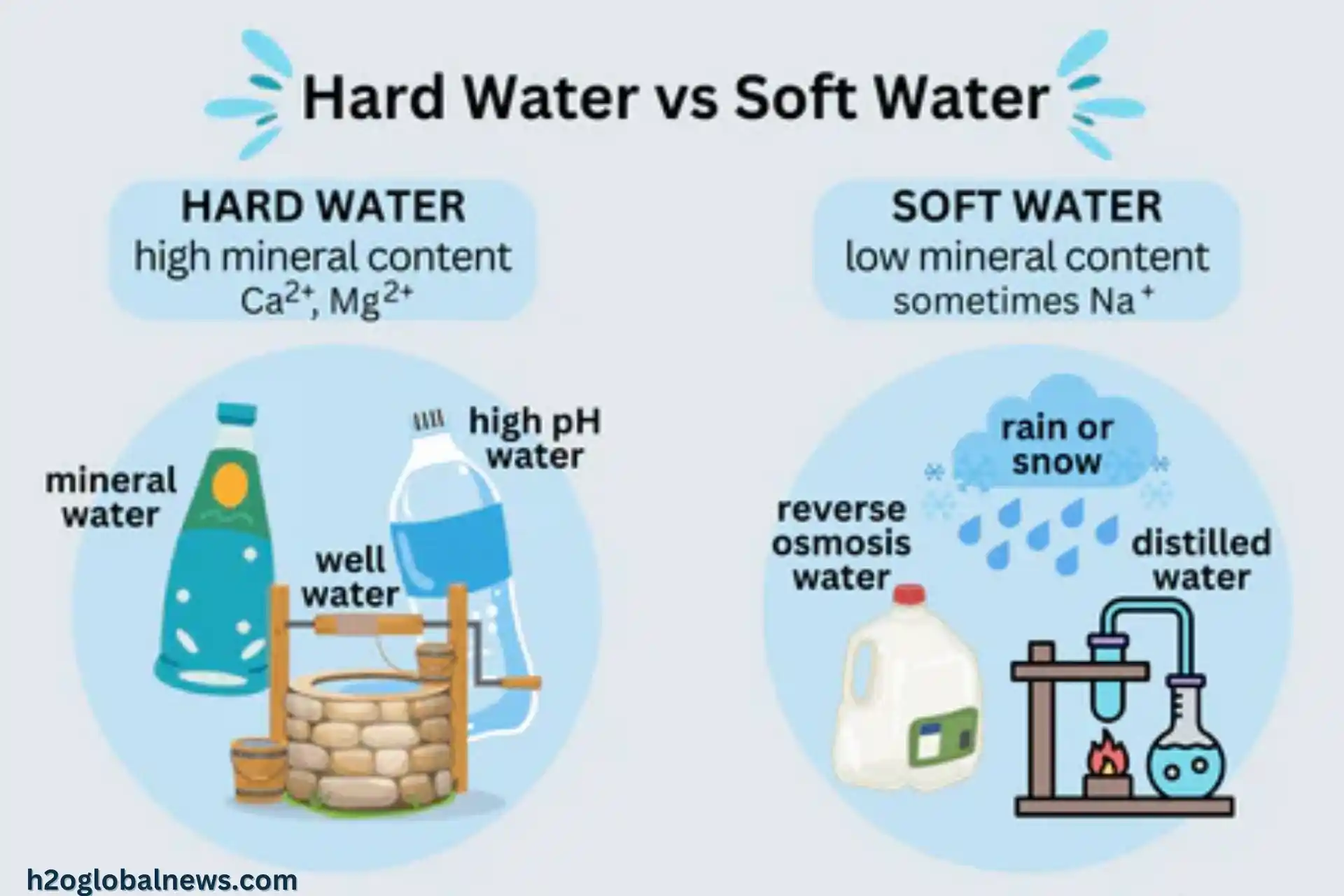
You may have heard of hard and soft water before, but do you know the difference between Hard and Soft Water? The distinction comes down to minerals – specifically, calcium and magnesium. Hard water contains many minerals, while soft water has little to none. For most people, the type of water that flows from their taps is determined by the local geology and water source. However, many homeowners install water softeners to convert hard water into soft.
Why does it matter? Hard and soft water affect household tasks in different ways. Hard water can leave spots on dishes, making it difficult to get soap and shampoo lather. Soft water, on the other hand, creates lots of lather and prevents spotting. It may also extend the life of water-using appliances. Understanding the difference between hard and soft water helps determine if a water softener could benefit your home.
Difference Between Soft and Hard Water
Hard water contains high amounts of calcium and magnesium ions, while soft water has little or none of these minerals. Calcium carbonate (CaCO3) level determines whether water is considered hard or soft.
Water hardness is measured in grains per gallon (gpg). Soft water has 0 to 3 gpg, slightly complicated is 3 to 7 gpg, moderately challenging is 7 to 10 gpg, and complex is 10 gpg or more. Areas with limestone bedrock often have hard water due to the natural calcium deposits. Soft water comes from surface sources like lakes, rivers, and reservoirs where the CaCO3 has not been built up.
The difference between the two types impacts several factors:
- Soap lathering: Hard water prevents soap from lathering easily due to chemical reactions with the minerals. More soap is required to achieve the same level of suds. Soft water produces lather much more readily with less soap.
- Scale buildup: Pipes and water heaters in challenging water areas accumulate mineral buildup over time, reducing efficiency and lifespan. Water softeners can help prevent this by exchanging the ions.
- Staining: Hard water can leave stains on dishes, showers, laundry, and other surfaces, as the excess minerals remain after the water has evaporated. Soft water does not leave behind these stains.
- Detergent use: Less detergent is required for soft water since there are no hard minerals with which to react. Additional detergent is needed for hard water to allow for mineral interaction and achieve cleaning.
In summary, while complex and soft water are potable and safe to use, their chemical differences impact daily life in several ways. A water softener can help overcome the challenges of hard water by exchanging the excess mineral ions for sodium or potassium chloride.
Effects of Hard and Soft Water on Your Home
Hard water contains high levels of calcium and magnesium ions, while soft water has little or none of these minerals. The differences between the two types of water can significantly affect your home.
Hard water prevents soap from lathering easily and can leave behind soap scum in sinks and showers. It also leads to limescale buildup that clogs plumbing and reduces the efficiency of water heaters. To combat these issues, you may need to install a water softener that replaces calcium and magnesium with sodium or potassium chloride.
In contrast, soft water produces a rich lather and prevents limescale deposits but may corrode metal pipes or leach copper over time. Although soft water is considered more pleasant for washing and cleaning, the sodium in some softeners could be problematic for people on low-sodium diets.
Laundry washed in hard water may become dull and stiff. Soft water allows detergents to clean more effectively, resulting in softer, brighter clothes. However, soft water can cause colors to bleed and fade more quickly. A water softener and a quality detergent formulated for soft water can maximize the benefits and minimize the negatives.
Whether to soften your water is a complex decision with many trade-offs regarding health, plumbing, appliance lifespans, cleaning effectiveness, and costs. Understanding how hard and soft water uniquely impacts your home can help you make the best choice. By being fully informed, you can enjoy refreshingly clean water sustainably and responsibly.
Health Impacts: Hard water vs Soft Water
Hard water contains high amounts of dissolved minerals, especially calcium and magnesium, while soft water has had these minerals removed. The type of water supplied to your home can significantly impact your health.
Hard Water
Hard water can lead to mineral buildup in plumbing and appliances, reducing efficiency and lifespan. The excess calcium and magnesium in hard water may also pose health risks. Hard water is associated with an increased risk of eczema and other skin conditions in children. For most adults, hard water likely has limited direct health effects when showering and washing. However, the calcium and magnesium in hard water can interfere with the absorption of some minerals and medications. Hard water may also increase the risk of kidney stones in some individuals.
Soft Water
Soft water has had most mineral content removed through an ion exchange process or reverse osmosis. Soft water tends to feel more pleasant when showering and washing as it produces more lather and leaves skin and hair feeling softer. It eliminates issues with mineral buildup and may decrease risks associated with hard water, like eczema and kidney stones. However, completely removing minerals from water may have some disadvantages. Soft water is slightly more corrosive and can leach heavy metals like lead and copper from plumbing. Soft water may also require the addition of mineral supplements to address potential nutritional deficiencies from a lack of calcium and magnesium.
What makes water “hard” or “soft”?
Hard water contains high dissolved calcium and magnesium levels, usually over 4 gpg. Soft water has had most or all of these minerals removed, usually less than 1 gpg. The more minerals, the more complex the water. Hard water is not harmful to your health but can cause issues like limescale buildup in pipes and water heaters, reducing efficiency.
How to check if water is hard or soft? Soft vs. Hard Water
- Check with your local water utility for a water quality report. It should list the levels of calcium and magnesium, measured in gpg. Over 4 gpg is considered problematic.
- Look for signs like limescale deposits in kettles, water heaters, or faucets indicating hard water. Soap scum is also harder to rinse off.
- Test your water hardness using test strips or a digital meter. Most home improvement stores sell water testing kits.
Conclusion
You now have a solid understanding of the differences between soft and hard water. Although hard water is safe for most uses, soft water can make household chores and hygiene easier by producing more lather and preventing mineral buildup. For some, the choice comes down to personal preference and water quality needs. Either way, knowing about your home’s water supply empowers you to make the best choice.
With the various water treatment options available today, every household can enjoy fresh, clean water that suits their needs.
FAQs
1- What is soft water?
Soft water has very low levels of dissolved minerals, making it smooth to touch and easier to use with soap.
2- What is hard water?
Hard water is water that has high levels of minerals like calcium and magnesium, which can cause scale buildup on surfaces and make it harder for soap to lather.
3- Can hard water cause diarrhea?
Hard water is generally not linked to diarrhea. However, if someone is not used to the high mineral content in hard water, they might experience mild digestive upset, which is rare and usually temporary.
4- What is considered hard water?
Hard water contains high levels of dissolved minerals, primarily calcium and magnesium. It’s typically hard if it has more than 120 milligrams per liter (mg/L) of calcium carbonate (CaCO₃).
5- What softens water?
Water is softened by removing calcium and magnesium ions through a process called ion exchange or by using reverse osmosis systems. Water softeners use sodium or potassium to replace these minerals, making the water “softer.”
6- What does hard water look like?
Hard water is clear and colorless, but it can leave behind visible mineral deposits, like white, chalky stains on faucets, showerheads, or glassware. These deposits are caused by calcium carbonate buildup from the minerals in the water.