Water is often called the universal solvent because it can dissolve more substances than any other liquid. This plays a huge role in our daily lives and in nature. From mixing sugar in tea to transporting nutrients in our bodies, water, as the universal solvent, makes it all possible. In this article, we’ll discuss the science behind why water is such a good solvent and explore how its molecular structure makes it so effective at dissolving other substances.
Why Is Water Called the Universal Solvent?
In science, water is the universal solvent because it can dissolve more substances than any other known liquid. Now, this doesn’t mean water can dissolve everything. For example, it can’t dissolve oils or plastic. But it still outperforms all other liquids, making it the best at dissolving solids, gases, and even some liquids. Before we go further, let’s understand the basics of solute and solvent.
The solvent is the liquid that dissolves, and the solute is the substance being dissolved. Together, they make a solution. There are three major scientific reasons that explain the powerful dissolving property of water.
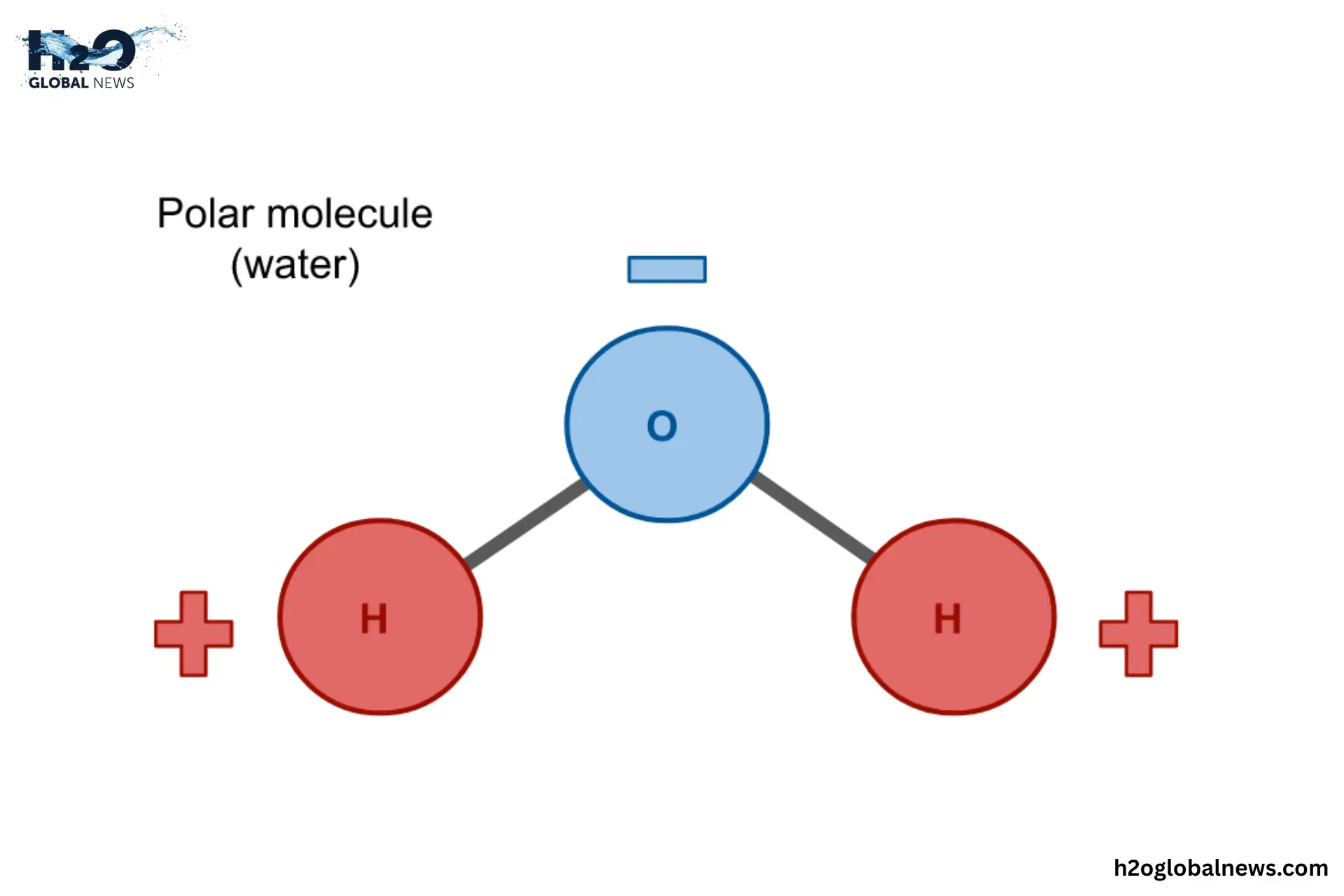
1- Water Is a Polar Molecule
A water molecule (H₂O) is made up of two hydrogen atoms and one oxygen atom. But the way these atoms are arranged is not even. Oxygen is stronger than hydrogen and pulls the electrons closer to itself. This gives the oxygen side a slight negative charge, while the hydrogen side becomes slightly positive.
Water has a positive end and a negative end, like a tiny magnet. This makes water a polar molecule. Because of this polarity, water can surround other charged or polar molecules and pull them apart, breaking them into smaller pieces and dissolving them.
For example, when you put salt (NaCl) in water, the positive side of the water attracts the negative chloride ions, and the negative side attracts the positive sodium ions, pulling them apart and dissolving the salt. That’s why salt disappears in water; it’s not gone, it’s just broken up and spread evenly. So, this polarity is the key reason why water is considered the universal solvent.
In fact, according to the U.S. Geological Survey, water dissolves more substances than any other liquid, which is why it’s involved in nearly every chemical reaction in biology and the environment.
2- Water Forms Hydrogen Bonds
Another amazing thing about water is that its molecules form hydrogen bonds with each other and with other substances. This happens because of something called hydrogen bonding.
Hydrogen bonds are weak attractions between the positive hydrogen of one water molecule and the negative oxygen of another. This constant attraction creates a network that helps water trap and carry other particles with it. That’s why water is able to transport nutrients in our bodies, carry minerals in rivers, and move fertilizers through soil.
The way water interacts with substances can also depend on its type, distilled, mineral, or tap all of which have different properties and dissolved contents. Understanding these types of water gives more context to how water behaves as a solvent in various situations.
3- Water Has a High Dielectric Constant
The dielectric constant of a liquid measures how well it can separate positive and negative charges in a substance. Water has a very high dielectric constant, about 80 at room temperature. That’s way higher than most other liquids, like alcohol (around 25) or benzene (around 2).
Because a high dielectric constant means water is really good at weakening the forces between charged particles. This helps it pull ions (charged atoms) apart and keep them dissolved instead of letting them stick back together. So, this property makes water excellent at dissolving salts, acids, and many other chemicals.
Real Life Example of Water as a Universal Solvent
Water’s amazing ability to dissolve substances shows up all around us. To make this clearer, here are a few real-world examples of water doing its job:
- In Your Body: Blood plasma is over 90% water, and it dissolves glucose (sugar), oxygen, carbon dioxide, hormones, and nutrients to carry them around your body.
- In daily life: When you make tea, coffee, or soup, water dissolves flavors, colors, and nutrients from herbs, vegetables, and spices.
- In Nature: Rainwater dissolves minerals in the soil, which helps plants absorb them through their roots.
- In Cleaning: When mixed with soap or detergent, water helps break down dirt, grease, and bacteria from surfaces, clothes, or even your hands.
FAQs
Is water a solute or solvent?
Water is usually the solvent in most mixtures, meaning it dissolves other substances. It rarely acts as a solute.
Is water polar?
Yes, water is a polar molecule, meaning it has a slight positive and negative side, which helps it attract and dissolve solutes.
Can water dissolve oil or fat?
No, water can’t dissolve oil or fat because they are non-polar, and polar substances like water don’t mix with non-polar ones.
Conclusion
Water is called the universal solvent because of its unique ability to dissolve more substances than any other liquid. Its polar nature, hydrogen bonding, and high dielectric constant make it perfect for breaking down and carrying solutes. This property is important for transporting nutrients in our bodies and shaping the Earth’s ecosystems. While it doesn’t dissolve everything, especially non-polar substances like oil, water still stands unmatched in its dissolving power. That’s why scientists, researchers, and educators agree that water as a universal solvent plays an irreplaceable role in both nature and daily life.